34+ Moles To Grams Calculator
Check how moles are relevant for solutions with our molarity calculator. And here is how you should.
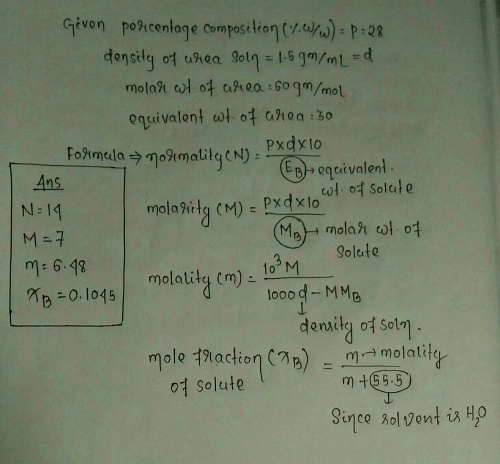
Find Out Mole Fraction Of 28 W W Solution Of Urea Which Have Density 1 5gm Ml Also Find 1 Molality And Normality Edurev Neet Question
Thus one mole of CO 2 weighs 4401 grams.

. How to calculate moles from grams. This means that one mole of carbon atoms weighs approximately 1201 grams. Web Calculate the molar mass of the substance.
Make sure you have a periodic table and a calculator handy. Therefore we would expect the corresponding mass to be about. Quantity of the substance moles.
Enter the moles and formula weight in the input field. N mM where m is the Mass of the element and M is the Molecular weight. Following the steps from Figure PageIndex2.
Moles of oxygen 64 grams 3200 grams per mole 2 moles. Web The given number of moles is a very small fraction of a mole 10 4 or one-ten thousandth. Weigh out 117text g 117g of sodium chloride.
Web If we have 64 grams of oxygen the number of moles would be calculated as follows. Multiply step one by step two. Number of grams Number of moles.
Web Mass 36 grams. Now click the button Solve to get the conversion value. Web Choose calculator 3.
Using the factor 1. Converting grams to moles. Web In practice we could use this information to make our solution as follows.
Web An online mole calculator helps you to calculate the number of moles of a substance based on the molecular weight also called molar mass and the quantity of that material. The number of grams of KClO3 will be 30637. Finally the conversion from moles to grams.
Web Multiply the given number of moles 250 mol by the molar mass 122548 gmol to get the grams. Mass of the substance grams. Web Converting grams to moles allows us to compare single atoms reacting with single atoms.
It is based on the molar mass of the. Formula Weight daltons Moles. Web 1201 2 1600 4401.
Web Convert moles to grams and grams to moles. How much is 1 mole to grams. A moles to grams calculator is a tool that helps convert a given amount of a substance from moles to grams.
Web Test Series. Web The molar mass of carbon C is approximately 1201 gmol. Transfer the sodium chloride to a.
The solution technique can also be. Molar Mass H2O 18 gmol. Web To convert moles to g we multiply the moles by the molar mass also known as the molecular weight.
Web To calculate the number of moles of an element use the formula. N fracmM n frac2358443 n. Moles H2O Mass g Molar Mass gmol 36 g 18 gmol 2 moles.
Convert 02 moles of Sodium. Web Consider you have 23 grams of NaCl. Web This Mole Calculator finds the quantity of a substance in moles and molar mass of the substance using its chemical formula and known mass of the substance in grams.
So there are 2 moles of water in 36. Choices and results Convert between moles and grams Moles from grams. Grams Moles x Molar Mass.
This means that 64 grams of. This relation provides a conversion factor to go from grams to moles. Web we can construct the appropriate conversion factor for determining how many grams there are in 000655 mol.
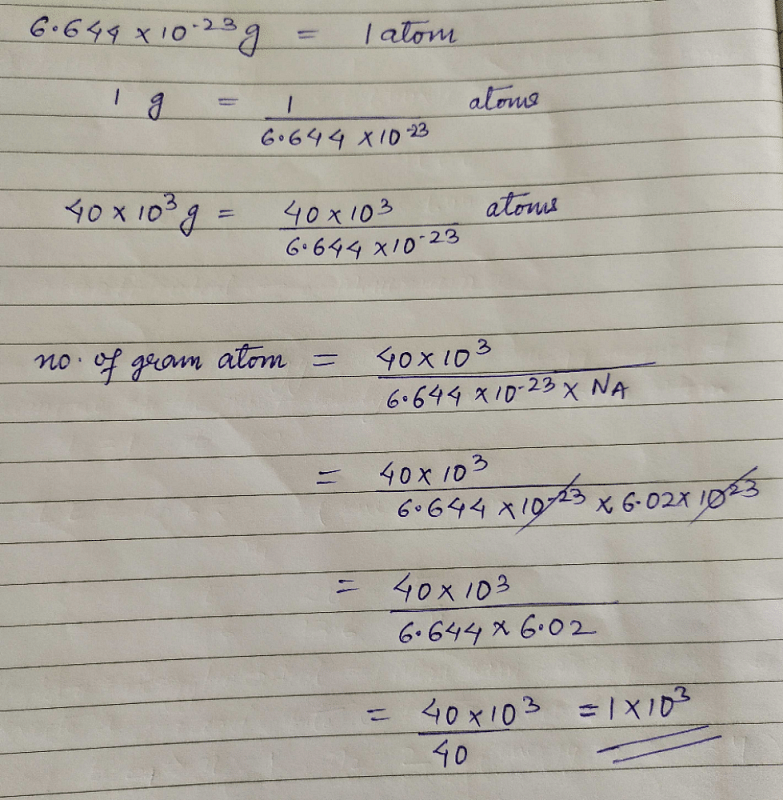
One Atom Of An Element X Weights 6 644 10 23g Calculate The No Of Gram Atom In 40kg Of It Edurev Neet Question

Moles To Grams Calculator Converter

Converting Grams To Moles Formula Calculation Examples Video Lesson Transcript Study Com

Calculate The Number Of Sulphur Molecules In A 300g Sample Of Sulphur S8 Edurev Class 9 Question
Solved How Many Moles Of O2 Are Needed To Produce 34 7 G Of Fe2o3 How Course Hero
How To Calculate The Tr Of Vam Quora
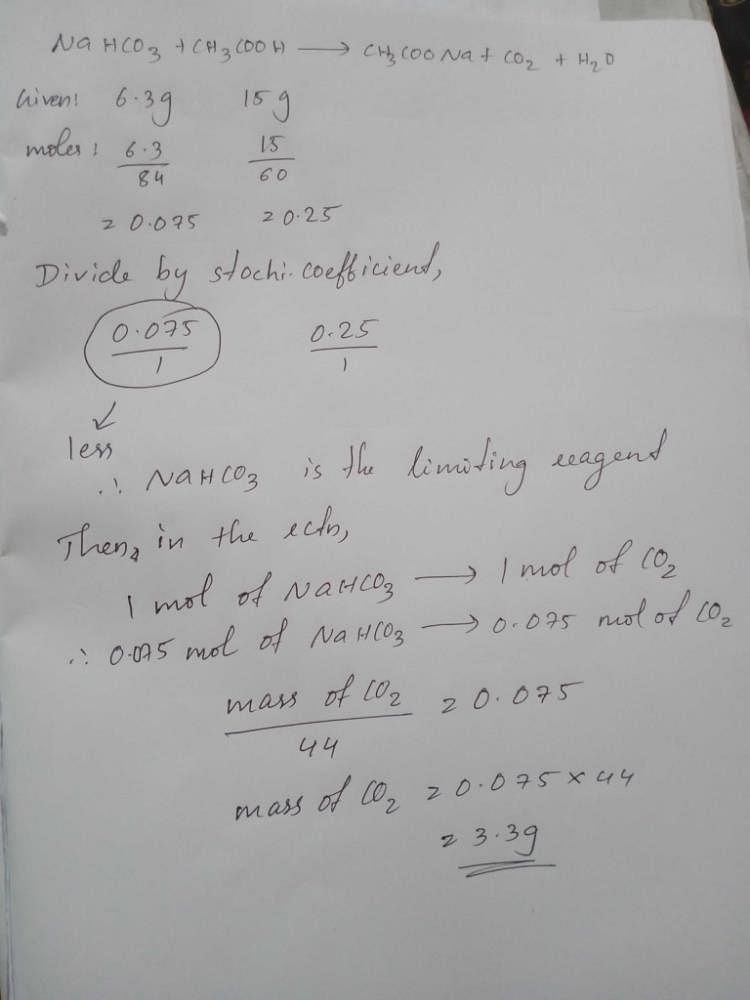
If 6 3 G Of Nahco3 Are Added To 15 0 G Ch3cooh Solution The Residue Is Found To Weigh 18 0 G What Will Be The Mass Of Co2 Released In The Reaction A 4 5 Gb 3 3

Chapter 4 The Mole And Stoichiometry Ppt Download
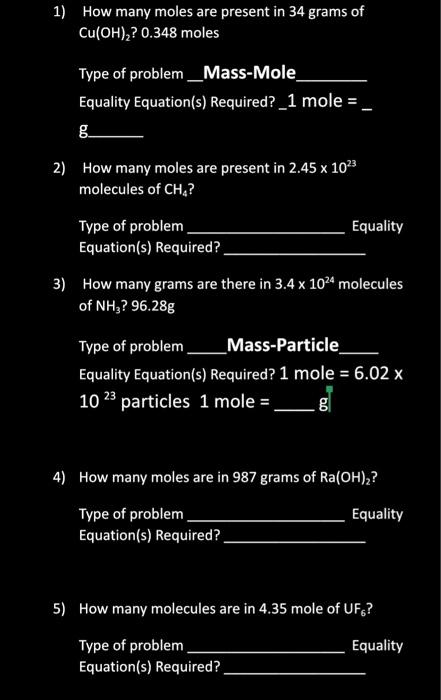
Solved 1 How Many Moles Are Present In 34 Grams Of Chegg Com
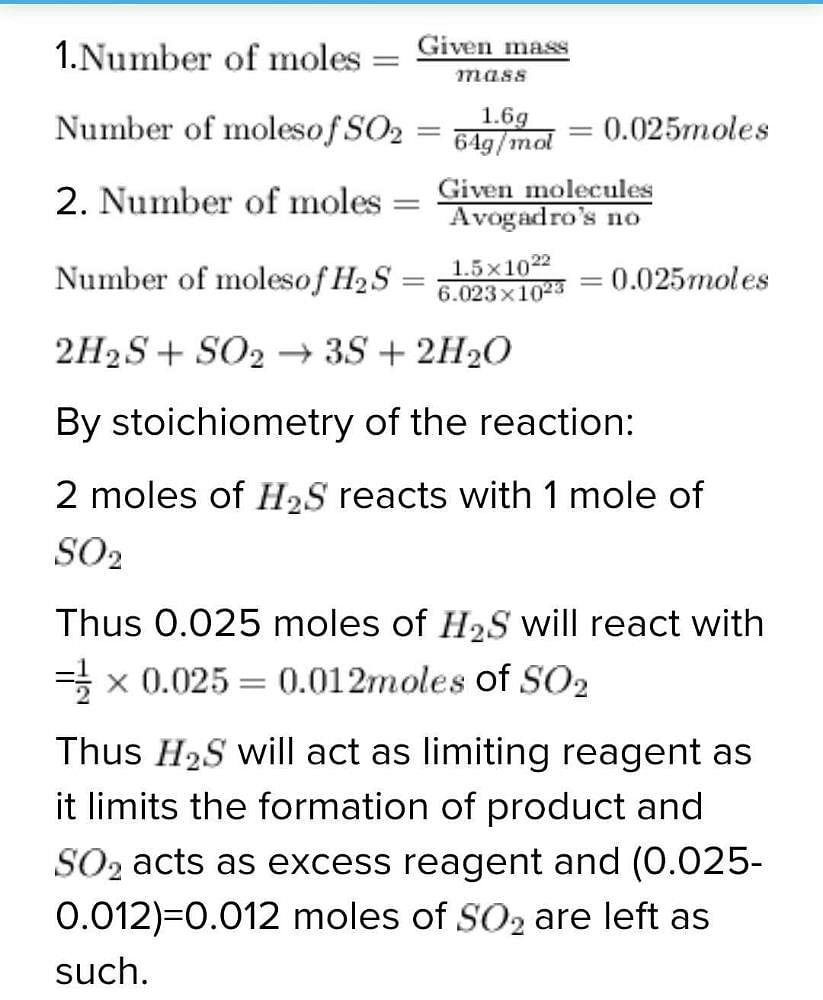
If 1 6 Gm Of So2 And 1 5 10 22 Molevules Of H2s Are Mixed And Allowed To Remain In Contact In Aclosed Vessel Until Tue Reaction Proceeds To Complete Whuch Is True Edurev Neet Question

Molar Mass Of A Substance 1g Of Which When Dissolved In 100g Of Water Gave A Solution Whose Boiling Point Is 100 1oc At A Pressure Of1 Atm Kbof Water 0 52 K
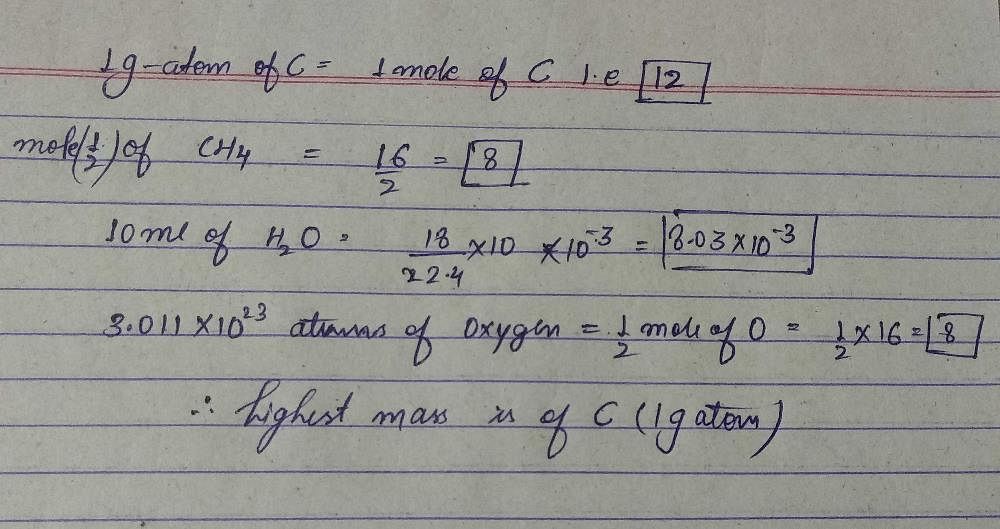
Which Of The Following Has The Highest Mass A 1 G Atom Of Cb 1 2 Mole Of C 10 Ml Of Waterd Atoms Of Oxygencorrect Answer Is Option A Can You Explain This Answer Edurev

Calculate The Number Of Sulphur Molecules In A 300g Sample Of Sulphur S8 Edurev Class 9 Question

Pogil Stoichiometry Submit Work Here Jun 11 2020 At 507 Pm Png Name Ave Munoz Date Period Model 2 Mole Mass Stoichiometry 2 Step Example Course Hero

The Mole And Stoichiometry Ppt Download

Moles To Grams Converter Calculator Moles To Gram Conversion
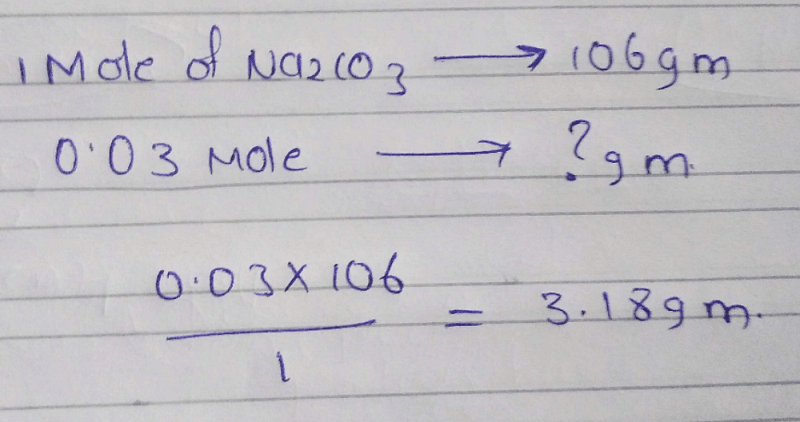
0 03 Mol Atom Of Na2co3 Has How Many Grams Edurev Neet Question